Why Can Alloys Not Be Described Using Chemical Formulas
There are two kinds of wrought alloys heat-treated or work-hardened. 1 atom exchange or 2 interstitial mechanism.
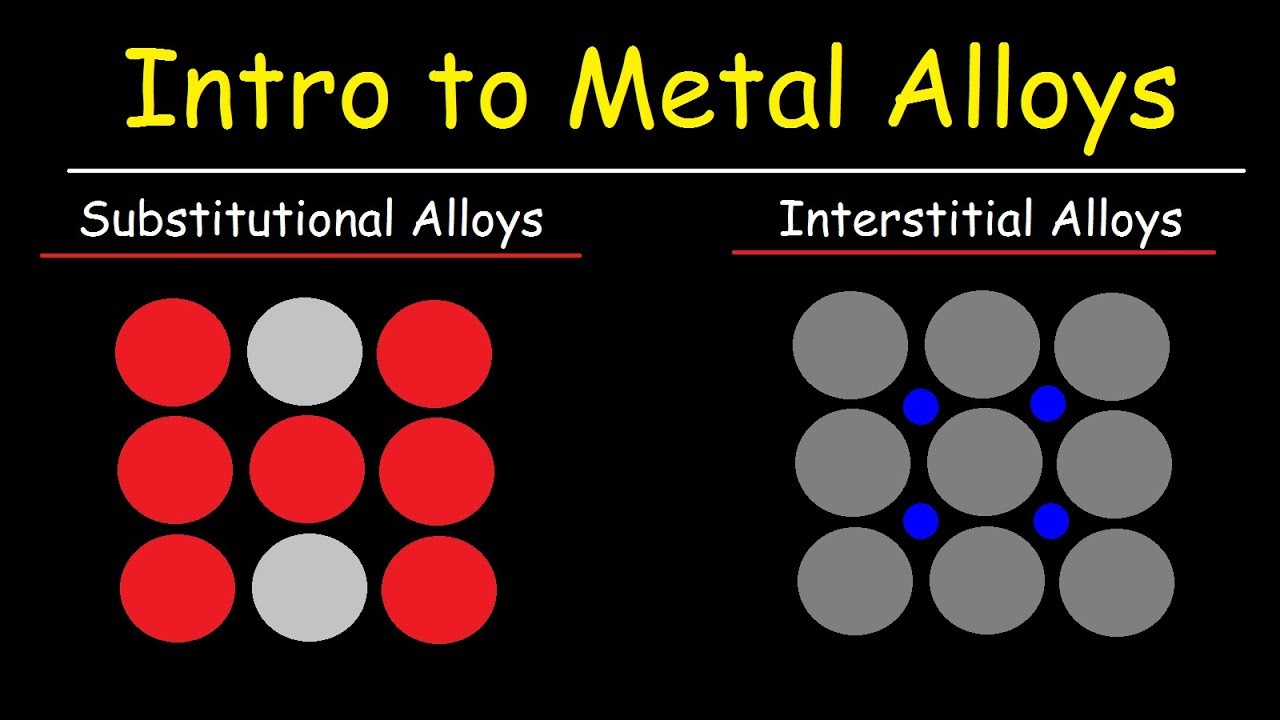
Metal Alloys Substitutional Alloys And Interstitial Alloys Chemistry Basic Introduction Youtube
The meaning of the term alloy is a substance formed from the combination of two or more metals.

. An alloy has different properties from the metals it. It offers supreme resistance to liquid. Alloys can be described using chemical formulas.
They are often thought of as physical mixtures of metals. Which of the answer choices correctly explains why alloys cannot be described using chemical formulas. Alloys have a structure that lacks covalent bonds.
Sometimes good results can be obtained after a brief etchclean in a 10 persulphate pre-etch. A chemical compound has a fixed composition. In alloys the atoms are of varying sizes.
Which of the answer choices correctly explains why alloys cannot be described using chemical formulas. This is an alphabetical list of alloys grouped according to base metal. Gold can be made to alloy with almost all other metals but most of the bodies thus formed are of little or no practical importance.
Alloys can also have properties. Very useful for gold alloys. The present work is.
The measure of strength of the chemical bond. Alloys dont have fixed formulas because their composition is variable. CeCaF2 does not mean the respective mineral is composed from ceCaF2 molecules but it means the ratio of ceCa2 and ceF- ions is in the ratio.
FeB binary alloys constitute the basis of many Fe-based metallic glasses with superior glass forming abilities and soft magnetic properties. This makes it difficult for the layers of atoms to move thus increasing its durability and. Nium persulphate and 10 g potassium cyanide.
Alloys have a structure that lacks covalent bonds. Alloys have a network structure. Which of the answer choices correctly explains why alloys cannot be described using chemical formulas.
Alloys are mixtures instead of compounds. Alloys are mixtures with undefined proportions. What is bond energy.
Why can alloys not be. Chemistry 19072019 0710 1230bering. When a molten metal is mixed with another substance there are two mechanisms that can cause an alloy to form.
This is due to their atom arrangement. Alloys will never rust b. An alloy is variable.
Alloys are simply described by the percentage of. 3003 like the other 3xxx alloys as well as 5052 another common alloy that can sometimes be used. See answer 1 Because an alloy is not a chemical compound.
Tin zinc arsenic and antimony unite with gold. Why can alloys be more useful than pure metals. Alloys do not have a chemical formula as they are not compounds with a stoichiometric relationship likely ionic or covalent bonds.
The Monel alloy 400 is used in hydrofluoric acid alkylation systems and in the development and handling of hydrofluoric acid. An alloy is a substance made by melting two or more elements together at least one of them metal. A alloys are mixtures with undefined proportions.
Theres more on the alloys used in British coins here. Select one or more. Today theyre copper-plated with cheaper steel forming the core of the coin.
An alloy crystallizes upon cooling into a solid solution mixture or. Why can alloys not be described using chemical formulas. Alloys are homogeneous mixtures they are not compounds like others and hence donot have formula.
Properties of alloys might be superior than the individual metals in the alloy c. The force that holds atoms or ions together in a compound. What is a Chemical bond.
Briefly explain why metallic. Alloys can also be formed from combinations of metals and other elements. An alloy is a material made by melting one or more metals together with other elements.
However when alloys are formed from their metals the. Alloys can be described using chemical formulas. Alloys have a network structure called a crystal lattice.
Alloy An alloy is a mixture of two or more elements at least one of which is a metal and the resulting mixture has metallic properties.

23 6 Alloys Chemistry Libretexts
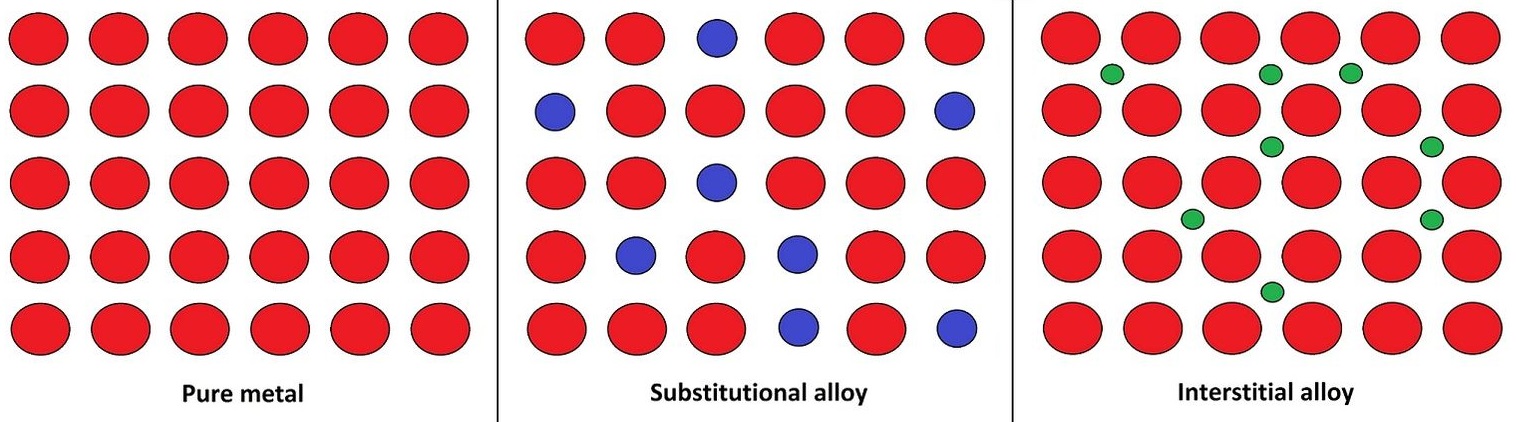
23 6 Alloys Chemistry Libretexts

Comments
Post a Comment